Prior to development of any formulations
the physiochemical properties of drug molecule should be known to the
formulator. Preformulation studies have a significant part to play in
anticipating formulation problems and identifying logical path in liquid, semisolid
and solid dosage form technology.
Prior to the development of
pharmaceutical dosage forms, it is essential that be valid fundamental physical
and chemical properties of the drug molecule and other divided properties of
the drug powder are determined.
Preformulation
is to make available and realize information regarding:
1)
The degradation process
2)
Any adverse conditions relevant to the drug
3)
Bioavailability
4)
Pharmacokinetics and formulation of similar compounds
5)
Toxicity.
Preformulation
influences -
a)
Selection of the drug candidate itself
b)
Selection of formulation components
c)
API and drug product manufacturing processes
d)
Determination of the most appropriate container closure system
e)
Development of analytical methods
f)
Assignment of API retest periods
g)
The synthetic route of API
h)
Toxicological strategy
i)
To establish its compatibility with common excipients by observing caking,
liquefaction, colour change, odour formation.
It
also gives directions for the development of formulation in choice of drug
form, excipients, composition, and physical form.
Introduction of
· Preformulation
testing is the first step in the rational development of dosage forms of a drug
substance.
· It
can be defined as a phase of research & development process for an
investigation of physicaland chemical properties of new drug substance alone
or in combination with other excipients in order to development of stable, safe
and effective dosage form. The possible interactions with the various
components intended for use in the final drug product are also considered. It
is an effort that encompasses the study of such parameters as dissolution,
polymorphic forms and crystal size and shape, pH profile of stability, and drug
– excipient interactions, which may have a profound effect on a drug’s
physiological availability and physical and chemical stability.
Objectives of Preformulation
The
overall objective of preformulation testing is to generate information useful
to the formulator:
a.
To
formulate stable, safe and effective dosage form
b.
To
increased drug stability
c.
To
improve drug bioavailability
d.
Reduce
drug excipient incompatibility
e.
It
is important to have an understanding of the physical description of a drug
substance before dosage form development.
f.
It
is 1st step in rational development of a dosage form of a drug substance before
dosage form development.
Goals of
a.
To
establish the physico-chemical parameters of new drug substance.
b.
To
establish the physical characteristics
c.
To
establish the kinetic rate profile.
d.
To
establish the compatibility with the common excipient.
e.
To
choose the correct form of a drug substance.
Applications of
Preformulation
studies begins or shall be updated
·
Immediately
after the synthesis and initial toxicity screening of a new drug.
·
When
a newly synthesized drug shows pharmacological evidence that requires further
evaluation in man
·
When
formulation and dosage form changes are required
·
When
solid form changes of DS are required.
Before
beginning the formal preformulation programs the preformulation scientist must
consider the following factors:-
·
The
amount of drug available.
·
The
physicochemical properties of the drug already known.
·
Therapeutic
category and anticipated dose of compound.
·
The
nature of information, a formulation should have or would like to have.
Following
studies are conducted as basic preformulation studies; special studies are
conducted depending on the type of dosage form and the type of drug molecules -
·
Solubility
determination
·
pKa
determination
·
Partition
co-efficient
·
Crystal
properties and polymorphism
·
Practical
size, shape and surface area.
·
Chemical
stability profile.
Protocol
for Preformulation Studies
Outline
of principal areas of preformulation research
Preformulation
Parameters
|
Preformulation parameters
|
Method used
|
|
Organoleptic Properties
|
Colour and Odour Determination
|
|
Crystallinity & Polymorphyism
|
X-ray Diffraction Studies (Lachman,
1991)
|
|
Fine Particle Characterization
|
Microscopic Method (Lachman,
1991)
|
|
Solubility Profile
|
Equilibrium Solubility Method (I.P. 2007)
|
|
Solubilization
|
(Lachman, 1991)
|
|
Analytical Method Development
|
UV Spectroscopic Method, HPLC Method
|
|
Ionization Constant, pKa
|
Determination of Spectral Shifts by UV Spectroscopy
(Lachman, 1991)
|
|
Partition Coefficient
|
Using octanol / water,(Lachman, 1991)
|
|
Bulk Density
|
Tapping Method (Lachman, 1991)
|
|
Powder Flow Properties
|
% Compressibility Determination, Angle of Repose (Lachman,
1991)
|
|
Compatibility With Excipients
|
DSC (Stulzer and Rodriques et al., 2008)
|
|
Stability
|
Solution and Solid State Stability (PCT/US03/35012)
|
|
Stability Indicating Method Development
|
Forced Degradation Studies (Rao et al., 2009)
|
Organoleptic
properties
·
Colour: Stability problems, improve appearance
by including dye in body or coating
·
Taste: Palatability, flavours, and excipient
may be added.
·
Odour: Degradation products, e.g. Aspirin
stable form of drug to be used, flavours and excepients may be used.
Suggested
terminology to describe organoleptic properties of pharmaceutical powders
Colour Odour Taste
Off-White Pungent Acidic
Cream
Yellow Sulfurous Bitter
Tan Fruity Bland
Shiny Aromatic Intense
- Odourless Sweet
- - Tasteless
Purity
- Purity studies
are essential for further studies to be carried out safely.
- Impurities may
make a compound toxic or render it unstable.
- TLC, HPLC, GC
and Paper chromatography used.
- HPLC-Impurity
Index (II), Homogeneity index (HI).
- DTA, gravimetric
analysis and melting point by hot stage microscopy are other techniques.
- Impurity index (II):
Defined as the ratio of all responses (peak areas) due to components other than
the main one to the total area response.
- Homogeneity index
(HI): Defined as the ratio of response (peak area) due to main component to the
total response.
E.g.:
main component –retention time: 4.39min
-area
response: 4620
Impurities
– 7 minor peaks; area response: 251
- Total area response:
251+4620
Impurity
index: = 251/(4620+ 251)
= .0515
Homogeneity
index: = 1 - .0515
= .9485
Other
Tools in Assessment of Impurity
·
Differential
thermal analysis(DTA)
·
Thermogravimetric
analysis(TGA)
·
Differential
scanning calorimetry (DSC)
·
Powder
X-Ray Diffraction (PXRD)
Particle
size and shape
-
Various
chemical and physical properties of drug substances are affected by their
particle size distribution and shapes.
-
The
effect is not only on physical properties as well as biopharmaceutical
behavior.
-
It
also influence the flow and the mixing efficacy of powders and granules.
-
Fine
materials are relatively more open to attack from atmospheric oxygen, humidity,
than that of coarse material.
Particle
size determination
- Microscopy. E.g.
Light microscope, electron microscope.
- Anderson Pipette
- Sieving method
- Instruments
based on light blockage (HIAC) and blockage of electrical conductivity path (coulter
counter are available).
Common
techniques for measuring fine particles of various sizes
Technique Particle size
(mm)
Microscopic 1 - 100
Sieve > 50
Sedimentation > 1
Elutriation 1 - 50
Centrifugal < 50
Permeability > 1
Light
scattering 0.5 - 50
Shape
determination:
-
Microscopy
should be carried out to determine the ratio of longest to shortest dimension.
It is a shape factor.
Shape factor
Ø
Commonly
used shape factor converts volume of particle ‘v’ to its volumetric mean
diameter ‘av’
§
V=αv.av³
Ø
Shape
factor may be defined which converts the surface area ‘s’of a particle to its surface mean diameter ‘as’
§
S=αs.as²
Ø
Fractal
Dimensions are carried out by imaging techniques.
In
(N) = -n In(g)+q where: N= no. of
squares.
g=length of grid size.
n & q are
constants
Surface
area determination
-
It
is determined based on Brunaver Emitter Teller (BET) theory of adsorption.
-
Most
substances adsorb mono molecular layer of gas (Nitrogen) and temperature.
-
Air
adsorption and permeability methods
Crystallinity
and polymorphism
Crystal
habit and internal structure of a drug can affect bulk and physiochemical
properties
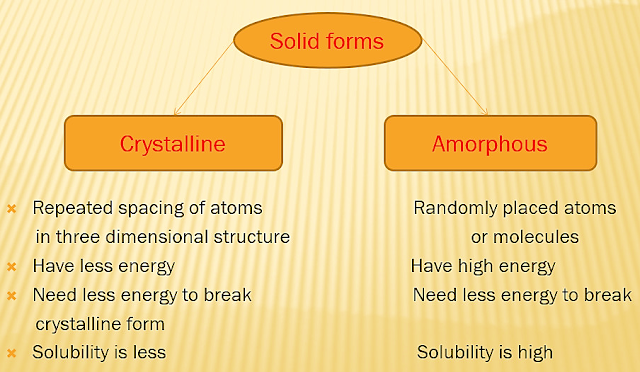
q HABBIT: Outer
appearance of crystal.
q Internal
structure
- Crystalline
- Amorpho
Characterization
of solid forms
Analytical
methods for characterization of solid forms
Method Material required per sample
a.
Microscopy 1mg
b.
Fusion
methods
1mg
(Hot stage microscopy)
c.
Infrared
spectroscopy 2-20mg
d.
X-ray
powder diffraction
500mg
e.
Scanning
electron microscopy 2mg
f.
Thermogravimetric
analysis 10mg
g.
Dissolution/Solubility
analysis mg to gm
Microscopy
q
All
substances are transparent examined under microscope – are either isotropic or
anisotropic
q
Isotropic
substances do not transmit the light – appears black – and have single
refractive index. E.g. Sodium Chloride
q
Anisotropic
substances – more than one refractive index – appear bright and brilliant color
– uniaxial and biaxial
q
Color
depends upon – thickness of crystal and diff. in refractive indices.
Thermal
Analysis
q Differential
Scanning Colorimetry (DSC) and Differential Thermal Analysis (DTH) measures the
heat loss or heat gain - resulting from physical or chemical changes.
q Two types of
processes
Endothermic: like fusion,
boiling, sublimation, vaporization, desolvation
Exothermic: like
crystallization, degradation
q Quantitative
measurement of these process have many application in preformulation study
including Purity, Polymorphism, solvation, degradation
X-Ray
Diffraction
·
Crystalline
materials gives characteristics pattern – by peaks in certain position &
varying intensities
·
Different
Polymorphs – different x-ray diffraction pattern due to crystal lattice.
·
Single
crystal x-ray analysis provides precise identification & description of a
crystalline substances.
Polymorphism
Substances can exist in more
than one crystalline form
·
Polymorphic
forms – diff. physical-chemical properties (incl. melting pt. & solubility)
·
Polymorphs:
-
Enatiotropic
-
Monotropic
·
Determination
method:
Thermodyanamically-van,
t Hoff plot (solubility vs temperature)
Directly
– by microscopic determination
Hygroscopicity
·
Many
substances, particularly water soluble salt form have a tendency to absorb
atmospheric pressure.
·
Change
in moisture level can influence chemical stability, flow ability, and compatibility.
·
It
can be monitored by Karl Fischer titration, TGA
Powder
Flow
·
The
pharmaceutical powders are classified as
-
Free
flowing
-
Cohesive
or non-free flowing
·
The
powder flow are affected by the changes in –
Density
Particle Size
Shape Free flowing
drug may become cohesive and
Electrostatic Charge necessitates an entirely new
formulation strategy
Adsorbed Moisture
Solubility
·
Solubility
> 1 % w/v
ð
no
dissolution-related absorption problem
·
Highly
insoluble drug administered in small doses may exhibit good absorption
·
Unstable
drug in highly acidic environment of stomach, high solubility and consequent
rapid dissolution could result in a decreased bioavailability.
·
The
solubility of every new drug must be determined as a function of pH over the
physiological pH range of 1 - 8.
Determination
of Solubility
Semi
quantitative determination:
Accurately
Quantitative determination:
General
Method of Increasing the Solubility
v
Addition
of co-solvent
v
pH
change method
v
Reduction
of particle size
v
Temperature
change method
v
Addition
of Surfactant
v
Complexation
Unique
Problems in Solubility Determination of Poorly Soluble Compounds
-
Solubility
could be over estimated due to the presence of soluble impurities
-
Saturation
solubility is not reached in a reasonable length of time unless the amount of
solid used is greatly in excess of that needed to saturation
-
Many
compounds in solution degrade, thus making an accurate determination of
solubility difficult
-
Difficulty
is also encountered in the determination of solubility of metastable forms that
transform to more stable forms when exposed to solvents
pH-Solubility
Profile
Poorly-soluble
weakly-acidic drugs:
pH = pKa + log
[(St - So)/So]
Poorly-soluble
weakly-basic drugs:
pH = pKa + log
[So/(St - So)]
where
So = solubility of unionized free acid or base
St = total solubility (unionized + ionized)
Process
of Solubilization
The
process of solubilization involves the breaking of inter-ionic or
intermolecular bonds in the solute, the separation of the molecules of the
solvent to provide space in the solvent for the solute, interaction between the
solvent and the solute molecule or ion.
Step
1:
Holes opens in the solvent
Step2: Molecules of
the solid breaks away from the bulk
Step
3:
The free solid molecule is intergraded into the hole in the solvent
Solubilization
can be enhanced by:
-
Use
more soluble metastable polymorph
-
Use
of complexation (eg.Ribloflavin-xanthinescomplex)
-
Use
of high-energy co-precipitates that are mixtures of solid solutions and solid
dispersions (eg. Griseofulvin in PEG 4000, 6000, and 20,000) in PEG 4000 and
20,000 -> supersaturated solutions in PEG 6000 -> bioavailability in
human twice > micronized drug
-
Use
of suitable surfactant
Partition
Coefficient
It
is the ratio of unionized drug distributed between organic and aqueous phase at
equilibrium.
P o/w = (C oil / C water) equilibrium
-
It
ratio of unionized drug in organic & aq. phase
-
It
measure lipophilicity
-
Major
role in drug transport
-
Analytical
separation
Ionization
Constant
·
The unionized species are more lipid-soluble
and hence more readily absorbed.
·
The GI absorption of weakly acidic or basic
drugs is related to the fraction of unionized drug in solution.
·
Factors affecting absorption:
-
pH
at the site of absorption
-
Ionization constant
-
Lipid
solubility of unionized species
“pH-partition
theory”
Henderson-Hasselbalch
equation
For
acids:
pH
= pKa + log [ionized
form]/[unionized form]
For
bases:
pH
= pKa + log [unionized
form]/[ionized form]
Determination
of Ionization Constant
1. Potentiometric pH-Titration
2. pH-Spectrophotometry Method
3. pH-Solubility Analysis
Dissolution
Diagram showing
dissolution and absorption of solid dosage form into blood circulation
PADDLE TYPE BASKET TYPE
2
types of systems to maintain uniform hydrodynamic conditions
1.
Static
disc dissolution apparatus
2.
Rotating
disc apparatus
Intrinsic
Dissolution
Film
Theory
The
dissolution of a solid in its own solution is adequately described by
Noyes-Nernst’s “Film Theory”.
-dW = DAK
(Cs - C)
dt h
Where
dW/dt = dissolution rate
A = surface
area of the dissolving solid
D = diffusion
coefficient
K = partition
coefficient
h = aqueous diffusion layer
Cs = solubility
of solute
C = solute concentration in the bulk medium
·
Intrinsic dissolution rate (mg/cm2/min) is
characteristics of each solid compound in a given solvent under fixed
hydrodynamic conditions
·
Intrinsic dissolution rate helps in
predicting if absorption would be dissolution rate-limited
·
1
mg/cm2/min --> not likely to present dissolution rate-limited
absorption problems
·
<
0.1 mg/cm2/min --> usually exhibit dissolution rate-limited
absorption
·
0.1
- 1.0 mg/cm2/min --> more information is needed before making any
prediction
Effect
of particle size of phenacetin on dissolution rate of the drug from granules
Solid
State Stability
·
For
identification of stable storage condition
·
Also
for identification of compatible excipient for a formulation
·
Extent
a product retains within specified limits and through its period of storage and
use
Stability
studies conducted in the preformulation phase:
·
Solid-state
of the drug alone
·
Solution
phase
·
With
the expected excipients
Photolytic
stability
·
Many
drugs fade or dropped on exposure light.
·
Exposure
of drug 400 and 900 foot-candles of illumination for 4 and 2 week periods
respectively is adequate to provide some idea of photosensitivity.
·
Resulting
data may be useful in determining if an amber colored container is required for
formulation.
Stability
to Oxidation
·
Drug’s
sensitivity to oxidation can be examined by exposing it to atmosphere of high
oxygen tension. Usually a 40% oxygen atmosphere allows for rapid evaluation.
·
Samples
are kept in desiccators equipped with three-way stop cocks, which are
alternatively evacuated and flooded with desired atmosphere.
·
The
process is repeated 3 or 4 times to ensure 100% desired atmosphere. Results may
be useful in predicting if an antioxidant is required in the formulation or if
the final product should be packaged under inert atmospheric conditions.
Solution
phase stability
·
As
compared with the dry form, the degradation is much rapid in solution form. It
is important ascertain that the drug doesn’t degrade when exposed to GI fluid.
·
The
pH based stability study, using different stimulator GI condition can be
designed.
·
A
poor solution stability of drug may urge the formulator to choose a less
soluble salt form, provided the bioavailability is not compromised.
Compatibility
studies
·
The
knowledge of drug excipients interaction is useful for the
formulation to select appropriate excipients.
·
The
described preformulation screening of drug excipients interaction requires only
5mg of drug in a 50% mixture with the excipients to maximize the likelihood of
obscuring an interaction.
·
Mixtures
should be examined under nitrogen to ultimate oxidation and paralytic effect at
a standard heating rate on DSC, over a temperature range, which will encompass
any thermal changes due to both the drug and appearance or disappearance one or
more peaks in themogrames of drug excipient mixtures are considered of
indication of interaction.
Flow
diagram to identify excipient compatibility with drug
Formulation
Recommendation
·
Upon
completion of preformulation evaluation of a new drug candidate, it recommended
that a comprehensive report be prepared highlighting problems associated with
this molecule
·
These
Reports re extremely important in preparing regulatory documents
Conclusion
q
Preformulation
studies have a significant part to play in anticipating formulation problems
and identifying logical path in both liquid and solid dosage form
technology.
q
By
comparing the physicochemical properties of each drug candidate with in a
therapeutic group, the preformulation scientist can assist:
-
the
synthetic chemist to identify the optimum molecule,
-
provide
the biologist with suitable vehicles to elicit pharmacological response and
-
advise
the bulk chemist about the selection and production of the best salt with
appropriate particle size and morphology for subsequent
processing.















Comments
Post a Comment